Ions are everywhere, playing crucial roles in everything from the batteries in your gadgets to the processes inside your body. But what exactly are ions, and why are they so important? Ions are atoms or molecules that have gained or lost electrons, giving them a positive or negative charge. This charge allows them to interact in fascinating ways, creating the foundation for many chemical reactions. Understanding ions can help you grasp how electricity works, why salt dissolves in water, and even how your nerves send signals. Ready to dive into the world of ions? Here are 15 intriguing facts that will electrify your curiosity!
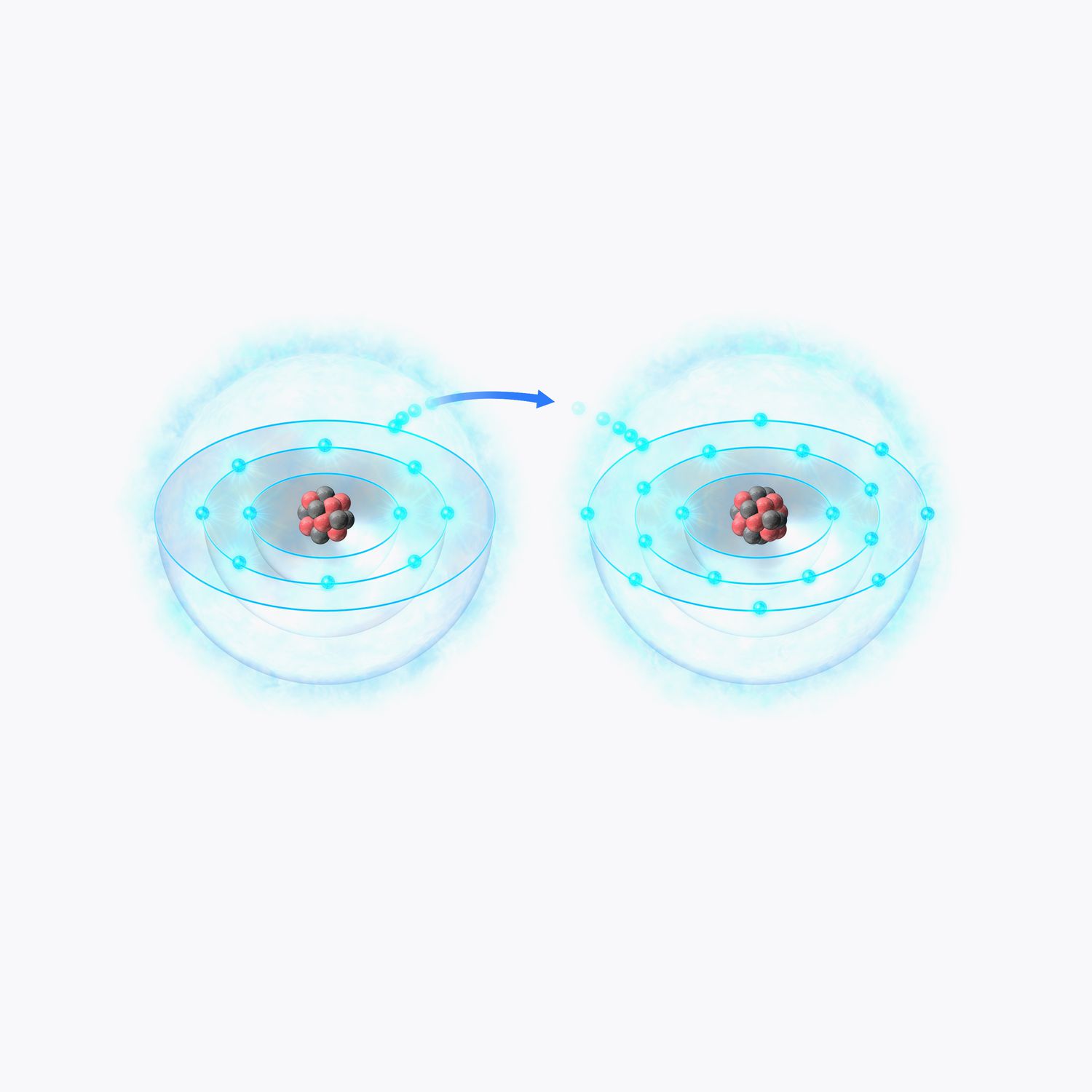
What Are Ions?
Ions are charged particles formed when atoms gain or lose electrons. They play a crucial role in various chemical reactions and biological processes. Here are some fascinating facts about ions.
-
Ions are either positive or negative. When an atom loses electrons, it becomes a positively charged ion called a cation. Conversely, when an atom gains electrons, it becomes a negatively charged ion called an anion.
-
Ions are essential for life. Many bodily functions depend on ions. For example, sodium and potassium ions help transmit nerve impulses, while calcium ions are vital for muscle contractions.
How Ions Form
Understanding how ions form can help explain their behavior in different environments. Here are some key points about ion formation.
-
Ionization energy matters. The energy required to remove an electron from an atom is called ionization energy. Elements with low ionization energy, like sodium, easily form cations.
-
Electron affinity is crucial. Electron affinity is the energy change when an atom gains an electron. Elements with high electron affinity, like chlorine, readily form anions.
-
Ions form in solutions. When salts dissolve in water, they dissociate into ions. For example, table salt (NaCl) separates into sodium (Na?) and chloride (Cl?) ions in water.
Types of Ions
Ions come in various types, each with unique properties and functions. Let's explore some different kinds of ions.
-
Monatomic ions consist of single atoms. Examples include Na? (sodium ion) and Cl? (chloride ion).
-
Polyatomic ions contain multiple atoms. These ions, like sulfate (SO?²?) and nitrate (NO??), have a group of atoms bonded together with an overall charge.
-
Complex ions are formed with central metal atoms. In complex ions, a metal atom is surrounded by other molecules or ions. An example is the hexacyanoferrate ion [Fe(CN)?]??.
Ions in Everyday Life
Ions are not just confined to chemistry labs; they are part of our daily lives. Here are some examples of ions in action.
-
Electrolytes in sports drinks. Sports drinks contain ions like sodium, potassium, and magnesium to help replenish electrolytes lost through sweat.
-
Water softeners use ions. Hard water contains calcium and magnesium ions, which can be removed using a water softener that exchanges them for sodium ions.
-
Batteries rely on ions. In batteries, ions move between electrodes to generate electricity. Lithium-ion batteries, for instance, use lithium ions to store and release energy.
Ions in Nature
Nature is full of ions, contributing to various natural phenomena. Here are some intriguing facts about ions in the natural world.
-
Ions create auroras. Auroras, like the Northern Lights, occur when charged particles from the sun interact with ions in Earth's atmosphere, causing beautiful light displays.
-
Ions are in the ocean. Seawater is rich in ions like sodium, chloride, and magnesium, making it a good conductor of electricity.
-
Ions in the soil affect plant growth. Plants absorb essential nutrients in ionic form from the soil, such as nitrate (NO??) and phosphate (PO?³?) ions.
Ions in Technology
Modern technology often harnesses the power of ions. Here are some ways ions are used in tech.
- Ion propulsion in space. Ion thrusters use ions to propel spacecraft, providing efficient and long-lasting propulsion for deep-space missions.
The Final Word on Ions
Ions play a huge role in our everyday lives. From the sodiumstrong> and potassium ions that help our nerves function to the calcium ions that keep our bones strong, these tiny charged particles are everywhere. They’re in the batteries that power our gadgets, the water we drink, and even the air we breathe. Understanding ions helps us grasp how chemistry and biology intersect in fascinating ways.
Next time you use a battery or drink a glass of water, remember the ions making it all possible. They might be small, but their impact is massive. Keep exploring the world of ions, and you'll uncover even more amazing facts about how they shape our world. Thanks for joining us on this journey through the electrifying world of ions!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


